38 fda approved drug labels
DailyMed DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical gases, devices, cosmetics, dietary supplements, and medical foods. The NLM provides DailyMed to the public and does not accept advertisements. FDA Resources: SPL, Other Prescription Drug Labeling ... FDA's Prescription Drug Labeling Resources. This website provides over 100 labeling resources for the Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for human prescription drugs, including biological products. FDA's Drug Guidances. Guidance documents represent the FDA's current thinking on a ...
Drugs@FDA: What's in a Drug Product Label? | FDA Information in Drug Product Labels. description of the drug. clinical pharmacology. indications (uses for the drug) contraindications (who should not take the drug) warnings. precautions. adverse ...

Fda approved drug labels
Pharmacogenetic Labeling of FDA-Approved Drugs - PMC The U.S. Food and Drug Administration recently marked 10 years since first updating the labeling for warfarin (often referred to as the "poster child" of pharmacogenomics) to include information regarding the potential impact of CYP2C9 and VKORC1 genetic variation on warfarin dosing requirements and risks. Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 03/23/2021: ORIG-1: Approval Label (PDF) Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration For prescription brand-name drugs, Drugs@FDA typically includes the most recent labeling approved by the FDA (for example, Prescribing Information and FDA-approved patient labeling when available),...
Fda approved drug labels. › scripts › cderDrugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 09/20/2021: SUPPL-60: Labeling-Package Insert, Labeling-Medication Guide Table of Pharmacogenomic Biomarkers in Drug Labeling - FDA The table below lists therapeutic products from Drugs@FDA with pharmacogenomic information found in the drug labeling. The labeling for some, but not all, of the products includes specific actions... FDA Drug Labeling Product Requirements, Guidance - PDG FDA's Guidance for Industry entitled "Help-Seeking" and Other Disease Awareness Communications by or on Behalf of Drug and Device Firms (January 2004) describes two types of drug labeling: FDA-approved labeling, and promotional labeling. An example of FDA-approved labeling is the Professional Package Insert (PPI). Why Is the FDA Seizing Baby Formula During a Baby Formula ... Hopefully the FDA's concern is truly about non-English labeling, different scoop sizes, recall alerts and transportation and storage safety—and not about protecting the interests of domestic ...
Fda Food Date Labeling - TheRescipes.info FDA Labeling Regulations The FDA labeling regulations are found in Title 21 of the Code of Federal Regulations (21CFR) parts 100-102; Close up on Food Labels - Information for California Food Processors (CA Dept. of Health, Food and Drug Branch, 2013) (PDF 653 KB) This publication explains general food label requirements. New Drugs - List of Latest FDA Approvals 2022 - Drugs.com Date of Approval: April 22, 2022. Treatment for: Rosacea. Epsolay (benzoyl peroxide) is a topical oxidizing agent for the treatment of inflammatory lesions of rosacea in adults. FDA Approves Epsolay (benzoyl peroxide) Cream for the Treatment of Rosacea - April 25, 2022. Epsolay FDA Approval History. Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 02/11/2021: ORIG-1: Approval Label (PDF) "Major" Drug Labeling Changes That Require FDA Prior Approval Some prescription drug label changes are considered sufficiently "major" that they require FDA prior approval - so says the FDA. If the FDA states that certain kinds of changes to drug labels need pre-approval, even for innovator drugs, then under Levine , Mensing , and Bartlett , product liability claims demanding those sorts of label ...
Drug Labeling Overview - Food and Drug Administration The openFDA drug product labels API returns data from these submissions for both prescription and over-the-counter (OTC) drugs. The labels are broken into sections, such as indications for use... OTC Drug Facts Label | FDA - U.S. Food and Drug Administration In the Federal Register of March 1999, the Food and Drug Administration published the OTC Drug Facts Label regulation. This regulation required most OTC drug products to comply with the new format... Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 03/18/2022: ORIG-1: Approval Label (PDF) Outbreak Investigation of Salmonella: Peanut Butter (May 2022) The FDA, along with CDC and state and local partners, are investigating a multistate outbreak of Salmonella Senftenberg infections linked to certain Jif peanut butter products produced at the J.M ...

Look for these FD&C (Food, Drug & Cosmetic) artificial dyes* on ingredient labels: Note: This is ...
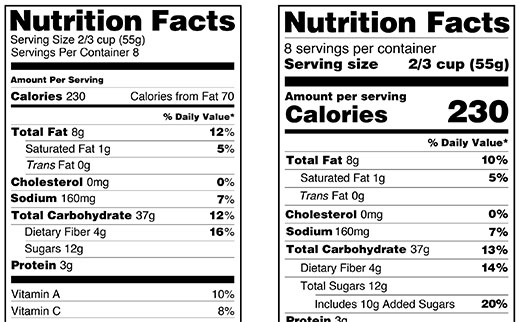
› it-really-fda-approvedIs It Really 'FDA Approved'? May 10, 2022 · The FDA does not approve individual food labels before food products can be marketed. But FDA regulations require specific labeling elements, including nutrition information, to appear on most ...
› animal-veterinary › productsApproved Animal Drug Products (Green Book) | FDA Feb 14, 2022 · Most FDA-approved animal drugs are included in a publicly available list of approved animal drug products. This list is called the Green Book for short, and FDA updates it in its entirety every month.
› scripts › cderDrugs@FDA: FDA-Approved Drugs - Food and Drug Administration * Drugs@FDA includes information about drugs, including biological products, approved for human use in the United States (see FAQ), but does not include information about FDA-approved products regulated by the Center for Biologics Evaluation and Research (for example, vaccines, allergenic products, blood and blood products, plasma derivatives, cellular and gene therapy products).
Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 04/26/2022: SUPPL-9: Label (PDF)
FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling documents....
animaldrugsatfda.fda.govAnimal Drugs @ FDA 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332)
Drug Safety-related Labeling Changes (SrLC) Patient Information - FDA approved patient labeling. Medication Guide - paper handouts that come with many prescription medicines. The guides address issues that are specific to particular drugs and drug classes, and they contain FDA-approved information that can help patients avoid serious adverse events.
Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels ... As of November 10, 2020, PharmGKB listed a total of five drug approvals with "recommended testing", of which two (i.e., azathioprine and thioguanine) were first approved prior to 2000 and to which biomarker information was added as part of subsequent labeling updates [ 62 ].
Drug Labels | FDA - U.S. Food and Drug Administration Drug Labels This is a partial collection of labeling submitted to the FDA Center for Veterinary Medicine (FDA CVM) by animal drug sponsors for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and...
› how-to-get-fda-approvalHow to Get FDA Approval | Registrar Labeling FDA Approved Products. Manufacturers of drugs and devices that do require FDA approval may include the phrase “FDA Approved” on the product’s labeling, as long as the manufacturer has received a letter from FDA confirming its approval. The FDA logo should not be used on a product’s labeling whether the product is approved or not.
Labeling Information | Drug Products | FDA - U.S. Food and ... For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web...
Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 03/23/2022: ORIG-1: Approval Label (PDF)
Prescription Drug Labeling Resources | FDA - U.S. Food and ... FDA's Prescription Drug Labeling Resources website provides over 150 labeling resources for the Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for...
FDA Issues 'Small Entity' Guidance for Importing ... To help small entities follow its 2020 final rule on importing prescription drugs from Canada, the FDA released a compliance guide yesterday explaining that U.S. importation programs must show "significant cost reductions" of the products to American consumers. Under the 2020 rule, the small entity — states, territories, Native American tribes, and license-holding pharmacists or ...
labels.fda.govFDA Label Search The drug labeling on this Web site may not be the labeling on currently distributed products or identical to the labeling that is approved. Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies described in monographs.
Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration Supplement Categories or Approval Type. Letters, Reviews, Labels, Patient Package Insert. Note. 12/21/2020. SUPPL-34. Efficacy-Labeling Change With Clinical Data. Label (PDF) Letter (PDF) 09/24/2020.
FDA-approved drug labeling for the study of drug-induced ... However, assessing the DILI potential of a drug is a challenge with no existing consensus methods. We proposed a systematic classification scheme using FDA-approved drug labeling to assess the DILI potential of drugs, which yielded a benchmark dataset with 287 drugs representing a wide range of therapeutic categories and daily dosage amounts.
.jpg)







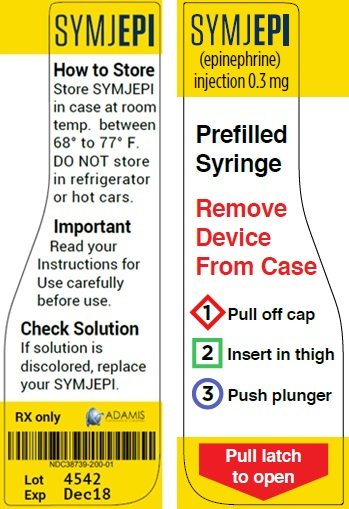
Post a Comment for "38 fda approved drug labels"